Businesses
Automation solutions from discovery to production at scale for producers of medical devices, medical diagnostics, pharmaceuticals and cell & gene therapies. Our expertise spans automated assembly, aseptic handling, fill/finish, advanced machine vision, and custom processes from depryrogenation to lyophilization helping customers scale with confidence, reliability and compliance

We deliver GMP compliant sterile drug production solutions in fill finishing isolators supporting vials, syringes, IV bags, bottles, and dry powders in pharmaceutical labs, R&D, and at-scale manufacturing. Our expertise also spans lyophilization, depyrogenation tunnels and washers, engineered for safety and efficiency. Whether for small clinical batches or large-scale commercial production, our solutions help pharmaceutical manufacturers deliver with confidence

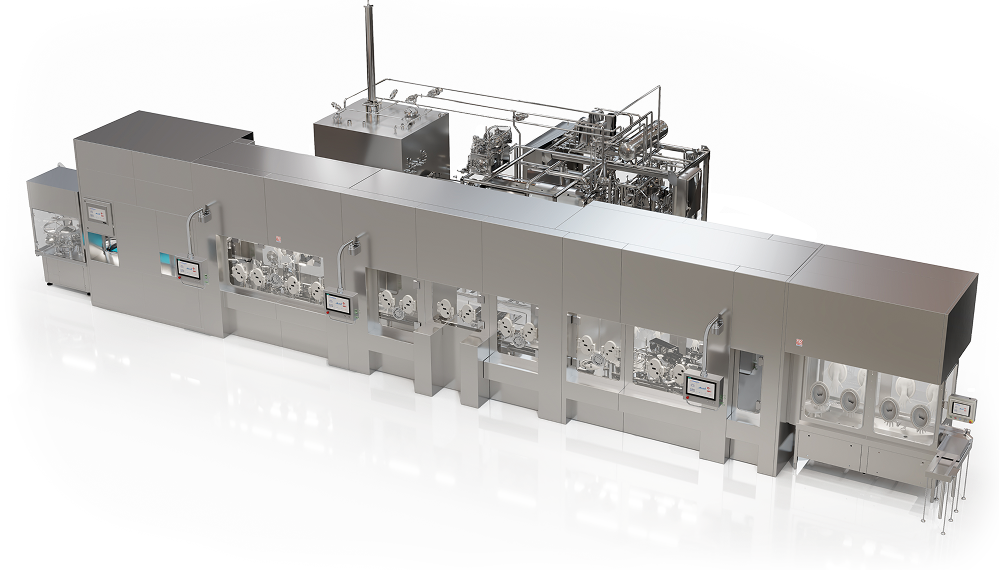

Precision manufacturing of medical diagnostics
From simple point-of-care test strips to microfluidic dispensing, we deliver advanced solutions engineered for quality assurance, consistent and reliable results. Our expertise in dispensing, assembly and inspection helps diagnostics manufacturers bring reliable technologies to market faster and with confidence
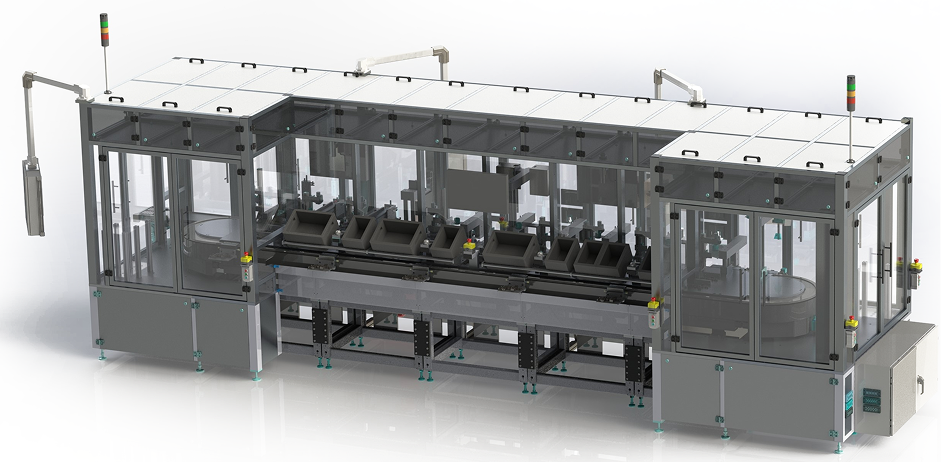
CUSTOM Automation for medical devices
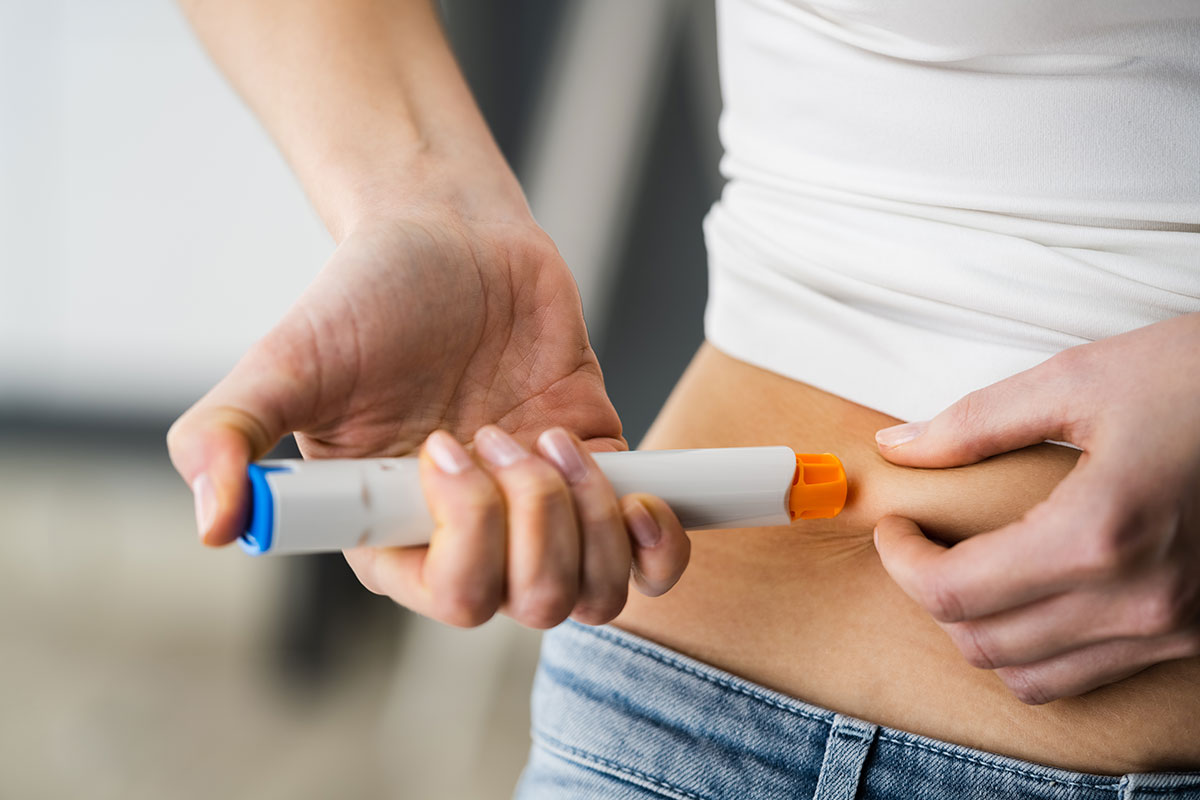
We provide precision automated assembly to ensure uncompromising product quality. From micro-assembly of smart wearables to complex drug delivery systems, our scalable, high-speed technologies deliver end-to-end traceability and customization, accelerating time to market while maintaining safety and reliability
Automation for cell & gene therapies
We deliver customizable GMP-compliant systems for ATMP, incorporating isolators, incubation systems, and lab automation. Our solutions minimize contamination risks from operators and external materials enabling sterile cell culture, testing, and validation under the highest cleanroom standards. Designed for both R&D labs and cell factories, they provide the flexibility and assurance needed to advance breakthrough therapies
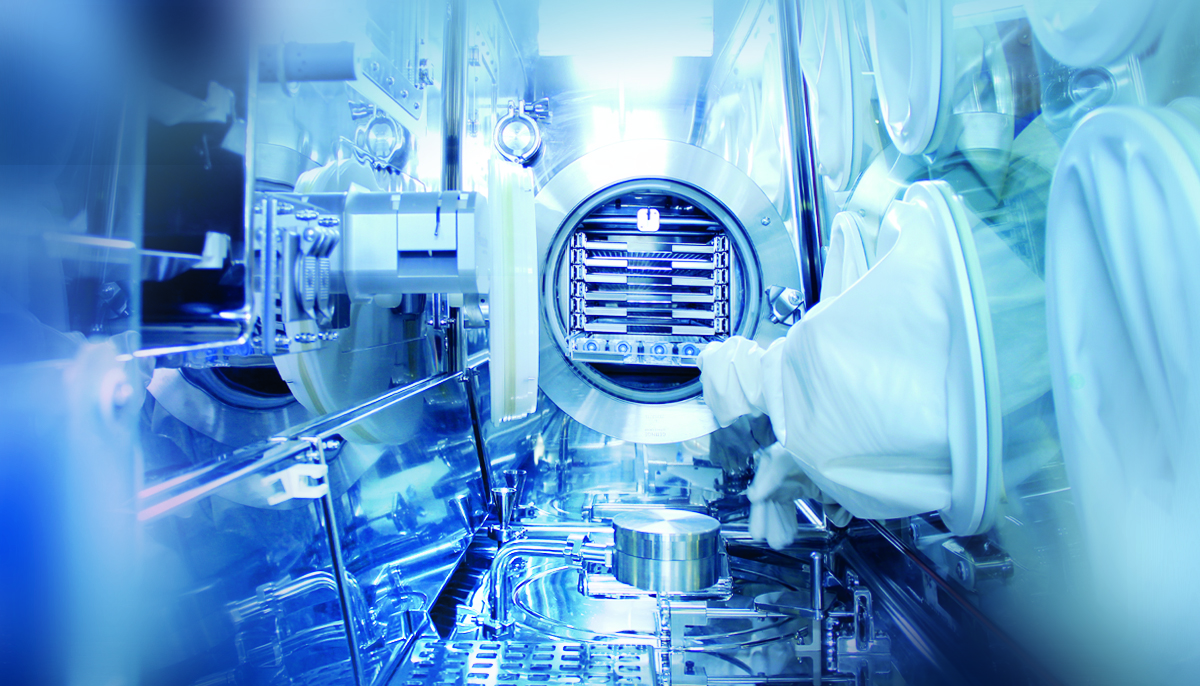
Automated manufacturing meets advanced Radiopharma solutions for nuclear medicine
Whether for clinical trial manufacturing or full commercialization, our solutions support scalable production while ensuring rigorous quality oversight at every stage. From shielded hot cells, laminar flow isolators, synthesis and dispensing systems, and automated vial and syringe filling solutions, to the capabilities that make your production fast, compliant, and end-to-end, the ATS LS Group helps safeguard patient safety while delivering consistent, high-quality throughput.
Partner with us to accelerate your life sciences journey.